Today we will take a closer look at another naturally occurring radioactive element, Lanthanum!
Lanthanum is a rare earth element and it’s the first element in the lanthanide series. It has an atomic number of 57 and was first discovered by a Swedish chemist, Carl Gustaf Mosander in 1839 but pure Lanthanum wasn’t obtained until 1923. Today, Lanthanum is most commonly used in the production of Tungsten electrodes, scintillators and in the past, it was added to some vintage lenses.

LaBr3(Ce) Scintilation crystals
Lanthanum Bromide scintillation crystals are well known for their incredible resolution which measures as low as 2.2% at 662 keV and since they have a relatively low price compared to other hi resolution detectors, they are a viable option for amateur gamma spectroscopy setups. These crystals however are not perfect, due to the Lanthanum content, they are slightly radioactive themselves which causes them to self generate a characteristic background spectrum that must be removed if measuring low activity samples.
Radioactivity and gamma spectroscopy
In nature, there are two isotopes of Lanthanum, La-139 (99.91% abundance) and La-138 which has an abundance of 0.09% and it is also radioactive.
Lanthanum 138 has a very long half-life of 1.02E+11 years and its activity cannot be easily detected with a conventional Geiger counter. To do that a large scintillator must be used since it is much more sensitive.
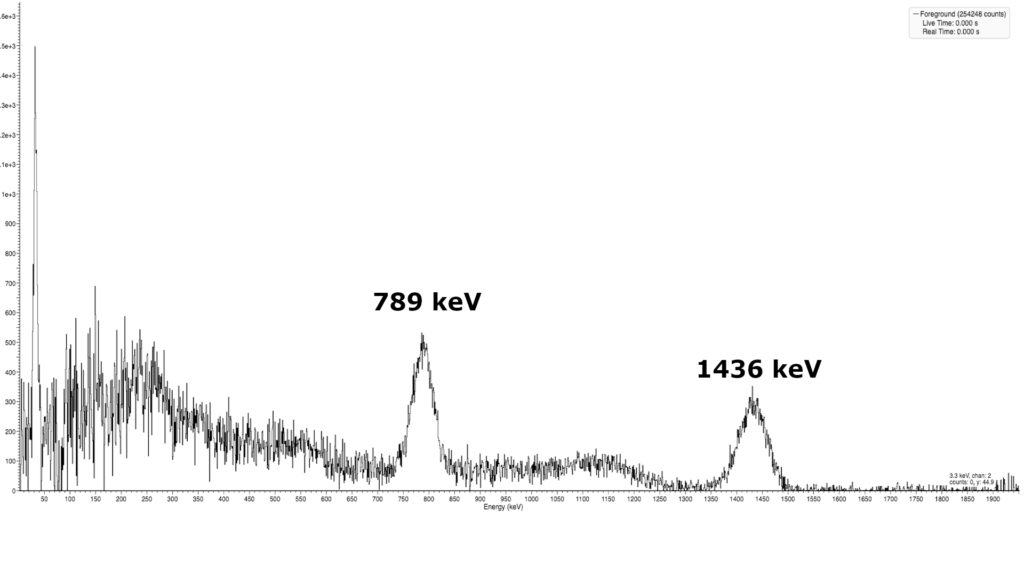
Unfortunately, my sample of Lanthanum is pretty small and my set-up isn’t sensitive enough to detect it so I reached out to my friend Gigabecquerell who has provided a gamma spectrum of his Lanthanum sample.
Lanthanum 138 decays through electron capture or by beta emission and in both cases, it also releases a gamma-ray at 789 keV and 1436 keV.
A gamma spectroscopy reviels two peaks at 789 keV and 1436 keV which are both caused by the decay of Lanthanum 138 through electron capture or by beta emission and in both cases a gamma ray is also released.