Welcome my fellow radiation nerds! Today we explore the radioactivity and the geology of the most common Thorium mineral – Monazite!
Monazite is a brownish phosphate mineral, primarily known for containing several of the rare earth elements, including Lanthanum, Cerium, and Thorium. It scores between 5.0 and 5.5 on the Mohs scale of mineral hardness and has a density of 4.6–5.7 g/cm³.
Compared to other Thorium minerals, such as Thorianite (70–80% ThO2) or Thorite (5–20% ThO2), Monazite contains much less Thorium, at around 6-7%. However, its widespread availability makes it the most common Thorium ore, accounting for approximately 12-15% of the world’s total Thorium reserves.
Monazite is most often located in placer deposits of sand and gravel. It’s primarily found in countries like India, Brazil, South Africa, Australia and China but it can be also found in many other places around the globe.
Fun fact: China is a major producer of Monazite due to its demand for rare earth elements, which also results in a significant surplus of Thorium. This surplus is playing a key role in driving the development of Thorium reactors as a potential alternative to uranium-based ones. It is also partially why there are so many radioactive “Scalar Energy Negative Ion” devices from China, as they contain trace amounts of Thorium from the Monazite extraction process.

Variations of Monazite
Monazite is not a single mineral, but rather a group of minerals with very similar structures and properties but with slightly different dominant elements. There are several variations of Monazite, with five main types. Three of these variations contain radioactive Thorium while another three contain Lanthanum (La) which does have a naturally radioactive isotope of La-138, but its radioactivity is extremely low and generally difficult to detect, especially in the presence of Th-232.
- monazite-(Ce), (Ce,La,Nd,Th)PO4 (the most common)
- monazite-(La), (La,Ce,Nd)PO4
- monazite-(Nd), (Nd,La,Ce)PO4
- monazite-(Sm), (Sm,Gd,Ce,Th)PO4
- monazite-(Pr), (Pr,Ce,Nd,Th)PO4

Mining History
Since Monazite is rich in the rare earth elements, it has been their primary source for many years.
In the 1880s, Carl Auer von Welsbach was looking for a supplier of Thorium to produce his newly invented thoriated gas mantles. One day he noticed that Brazilian ships used Monazite sand as ballast, and soon, Brazil became a key supplier of Thorium for the production of the gas mantles
For many years, Brazilian and Indian Monazite dominated the industry. However, after World War II, much of the mining activity shifted to South Africa.
Unfortunately, due to the radioactive waste produced during Monazite processing, it was eventually phased out in favor of Bastnäsite in the 1960s, which contains much less Thorium making it safer to work with.
In the recent years, the growing interest in Thorium reactors as a potential alternative to Uranium reactors has sparked renewed interest in Thorium. This shift could lead to an increased demand for Thorium and possibly bring Monazite back into commercial use.
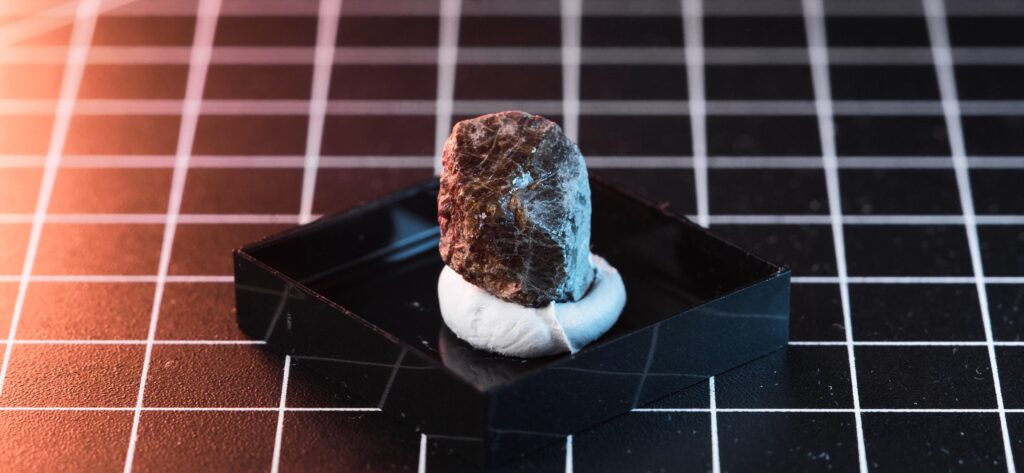
My Sample
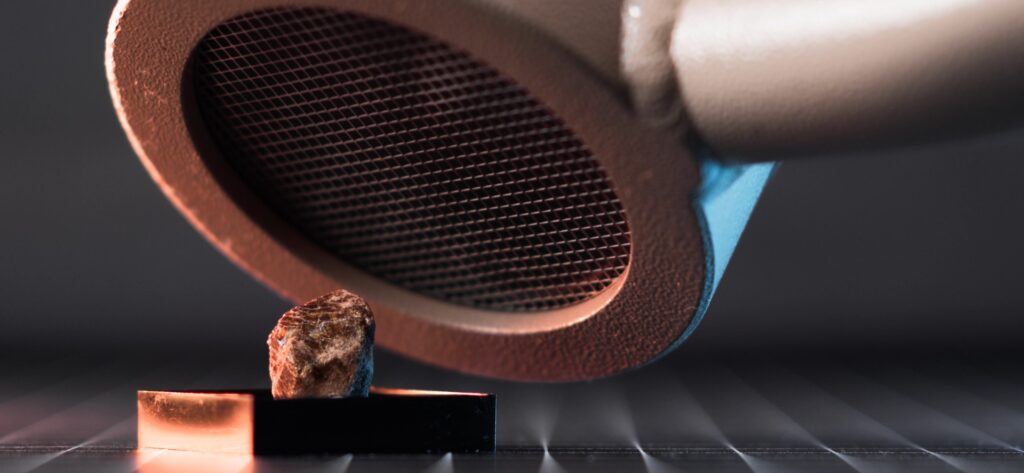
I acquired my sample at a recent mineral trade show. It’s the most common variation, Monazite-(Ce), and it comes from the Marijao Pegmatite region in Madagascar. Despite its small size, its radioactivity is easily detectable, reading around 2000 CPM on my Ludlum Model 3 with a 44-9 probe at a 1 cm distance.
Although I already know from its chemical formula that Thorium is present, I decided to do a Gamma Spectrum of the sample with my RAYSID gamma spectrometer. As expected, it revealed peaks characteristic of the Thorium-232 and its decay chain.
Conclusion
I’d love to hear about your experiences with Monazite or other Thorium-containing minerals. Do you have any in your collection? Let me know in the comments below!
Thank you so much for reading this post, I hope you enjoyed it and learned something new! If yes, please make sure to subscribe to the email list so that you get notified when new posts are added. Also feel free to check out my Ko-Fi page where you can donate a nice cup of radioactive coffee and support my work financially.
and remember, stay active!

2 Comments
marian · 19 October 2025 at 20:36
Hello,
Thank you for this very informative post. Today at a trade fair I came across a handful of samples of Monazit, Euxenit, Betafit, and Smaraskit. The seller was keeping the samples under lead foil which spooked me a little. So before buying them I wanted to do my due diligence about how to safely display the samples. Corect me if I am wrong but a 5 mm thick pmma or glass box should be enough to block the emited alpha and beta particles, or? How to you keep your samples?
allRadioactive · 21 October 2025 at 15:59
Having a few or even several radioactive minerals does not pose any risk. Make sure to wear single use gloves while handling radioactive minerals, always wash your hands and watch out for small particles that might have fallen off the mineral. I recommend you book called “introduction to radioactive mineral collecting”. It has many useful tips!