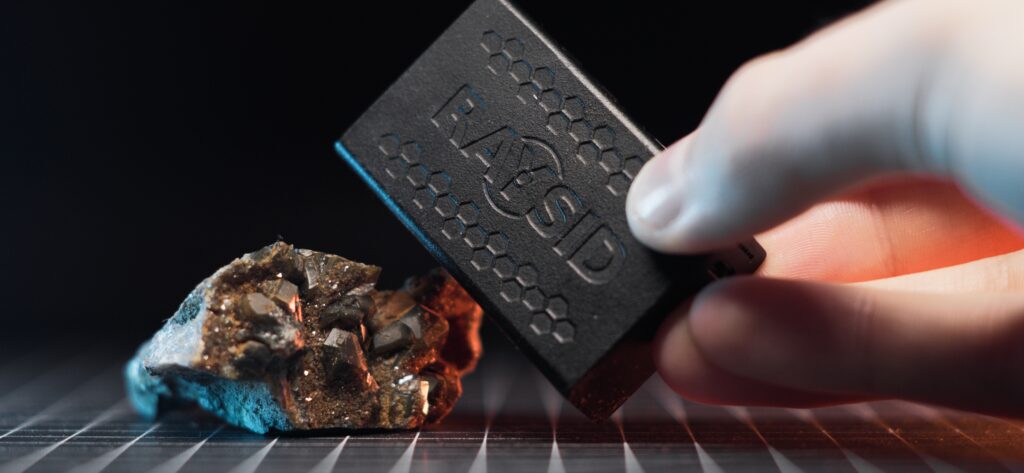
Adrianov’s Compass – Another Radioactive Artefact of the Cold War
Welcome back my fellow radiation nerds. Today we will take a look at another soviet, radioactive artefact from the Cold War, the Adrianov’s Compass!
The history of the compass
The Adrianov’s compass was designed by Vladimir Adrianov in 1907 for the Russian Imperial Army and since then, it served in various countries within the Warsaw Pact until it was eventually phased out in the late 1950s. Although originally intended for military use, the compass became widely adopted by scout groups and other paramilitary organisations.
These compasses used radium paint on the tips of the arrows and few other spots to make them glow in the dark, which also resulted in them being radioactive. Due to their old age, there is no visible glow left and under black light there is a little bit of green fluorescence but the radioactivity is easily detectable..
It is worth mentioning that only the models with metal bodies and orange paint on the arrows used radium. The ones with plastic, white bodies or without orange paint aren’t radioactive.
The built quality of the compass is very solid and there are no loose parts, which definitely helps with containing any bits of radium paints that might have fallen off. This being said, there is a little bit of radon gas leaking out.
On the back there is a logo of the factory in which the compass was manufactured and the year of production. Mine was made in 1956 by SZMO (Śląskie Zakłady Mechaniczno-Optyczne w Katowicach) in Katowice, Poland. Although it is 69 years old, it is still in a great visual condition.
Today these compasses can be easily found pretty cheaply at flea markets, especially in countries that were part of the Warsaw Pact.

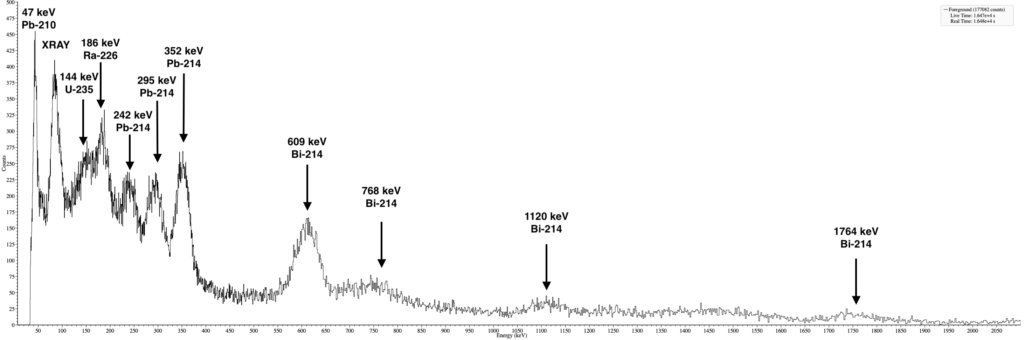
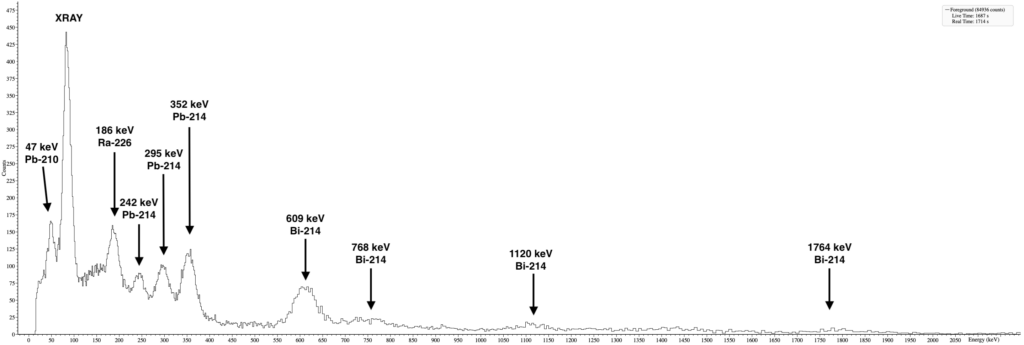
Activity and gamma spectroscopy of the compass
When measured with My Ludlum Model 3 with a 44-9 probe, I got a reading of 10k CPM at 1cm distance. The thick glass blocks pretty much all alpha and beta radiation and if removed, the activity from the radium paint will easily max out my meter at over 500k CPM but I strongly advise against opening any items containing radium paint.
The gamma dose from the compass is approximately 5.50 uSv/h when measured with my RAYSID at 1cm distance.
A quick gamma spectroscopy showed clear peaks for Ra-226 and its decay chain.
A few last words
Through out the history, various militaries used countless radioactive items from radium painted watches, airplane gauges to even helmet markers and while those items are definitely great collectables for anyone fascinated with military equipment of the 20th century, it is important to remember to always handle such items with most care, as Radium is a particularly nasty element, and the paint can easily fall apart and contaminate anything it touches. Remember that safety always comes first.
I want to hear from you! Do you have any radioactive watches or compasses? What other radioactive items should I explore? Let me know in the comments below!
Thank you so much for reading this post, I hope you enjoyed it and learned something new! If yes, please make sure to subscribe to the email list so that you get notified when new posts are added. Also feel free to check out my Ko-Fi page where you can donate a nice cup of radioactive coffee and support my work financially.
and remember, stay active!